Why is the Moon rich in Helium-3, a strategic resource? In what form is helium-3 stored on the Moon? How to mine Helium-3 in situ? Recent studies have shown that lunar glass plays a key role in trapping and preserving Helium-3 gas.
Helium-3, an isotope of Helium (the second element in the periodic table), has important applications in the fields of energy, scientific research. For example, as a fuel for controlled fusion, Helium-3 fusion produces 250 times the energy needed to mine it and 12.5 times the energy needed to fission Uranium-235 (about 20). The energy produced by 100 tons of Helium-3 fusion can supply the whole world for one year, and the process of Helium-3 fusion has no secondary radiation risk of neutrons, so it is cleaner and more controllable. In addition, Helium-3 is a key refrigerant for extremely low temperature environments, and is essential for cutting-edge research fields such as superconductivity, quantum computing, and topological insulators. However, Helium-4, the main element of helium on Earth, contains only about 0.5 tons of 3elium-3, which is far from enough to meet current demand.
Helium-3 is an important component of the solar wind, so the Moon irradiated by the solar wind over the years has a large storage of Helium-3. But why is the Moon rich in Helium-3, a strategic resource? In what form is Helium-3 stored on the Moon? There are no clear answers to these questions. Exploration of lunar resources, especially the content, distribution and mining of Helium-3, has become an inevitable trend and main task of international deep space exploration. Therefore, from the end of the 20th century, a new round of lunar “gold rush” was set off, bringing lunar exploration projects and scientific research to a new climax. But how to mine Helium-3 in situ and efficiently remains a scientific and technical challenge. Previous studies believed that Helium-3 was dissolved in lunar regolith particles, and extraction of helium-3 was limited by diffusion rate and required high temperature above 700℃, which was not only high energy consumption, but also slow, which was not conducive to mining in situ on the moon. Therefore, discovering the storage form of Helium-3 in lunar regolith is very important for understanding the issues that how the Moon captures Helium-3 and how to exploit and utilize Helium-3 resources in the future.
Recently, a joint team from Ningbo Institute of Materials Technology & Engineering of Chinese Academy of Sciences (CAS), Qian Xuesen Laboratory of China Academy of Space Technology, Institute of Physics (CAS) and Nanjing University has investigated the helium atoms in Chang 'E-5 lunar particles. It is found that there is a layer of amorphous glass on the surface of lunar ilmenite particles. By using high resolution transmission electron microscopy (TEM) combined with electron energy loss spectroscopy (EELS), they observed a large number of helium bubbles in the glass layer, with diameters of about 5~25nm, and most of the bubbles were located near the interface between the glass layer and the crystal. Inside the crystals, there are almost no helium bubbles. Because of the high solubility of helium in ilmenite, the researchers believe that helium atoms were first injected into the ilmenite lattice by the solar wind, and then gradually released due to the channel diffusion effect of the lattice. The surface glass has a disorderly accumulation of atoms that limits the release of helium atoms, which are trapped and gradually stored, forming bubbles.
Glassy materials have extremely high stability due to their special disordered atomic accumulation structure. For example, glassy amber can preserve biological specimens for hundreds of millions of years, and oxide glass can store nuclear waste for thousands of years. This work shows that ilmenite glass is also extremely stable, capturing and preserving rich helium-3 resources on the Moon.
This work shows that mechanical crushing can be used to extract helium-3 stored in bubble form at room temperature without heating to high temperatures. Moreover, ilmenite has weak magnetism and can be separated from other lunar particles through magnetic screening, which is convenient for mining in situ on the moon. Through further calculations, the researchers found that the helium atoms in the bubble have a number density of 50-192 He/nm3, which is extremely high pressure. Based on the amount of ilmenite on the Moon, the amount of Helium-3 stored in bubble form could be as high as 260,000 tons, which could meet the world's energy needs for 2,600 years if all of it were used for nuclear fusion. These results not only provide new insights into the enrichment mechanism of Helium-3 on the Moon, but also lay a theoretical foundation for the mining in situ and utilization of Helium-3 on the Moon in the future, which is of great significance to explore the effective utilization path of lunar resources.
This work was published in the journal “Material Futures” under the title “Taking advantage of glass: Capturing and retaining of the helium gas on the moon.” Materials Futures 1(2022)035101,DOI:10.1088/2752-5724/ac74af. This work was completed by the project team of lunar regolith physical properties research and comprehensive utilization led by Weihua Wang, Academician of Institute of Physics, CAS, Mengfei Yang, Academician of China Academy of Space Technology, and Zhigang Zou, Academician of Nanjing University. The lunar regolith sample number is CE5C0400. Junqiang Wang, Juntao Huo, Wei Xu, (Ningbo Institute of Materials Technology & Engineering, CAS) and Haiyang Bai (Institute of Physics, CAS) are the co-corresponding authors. Dr. Ao Li, Dr. Xiao Chen, Dr. Lijian Song and Dr. Guoxin Chen from Ningbo Institute of Materials Technology & Engineering, CAS are co-first authors.
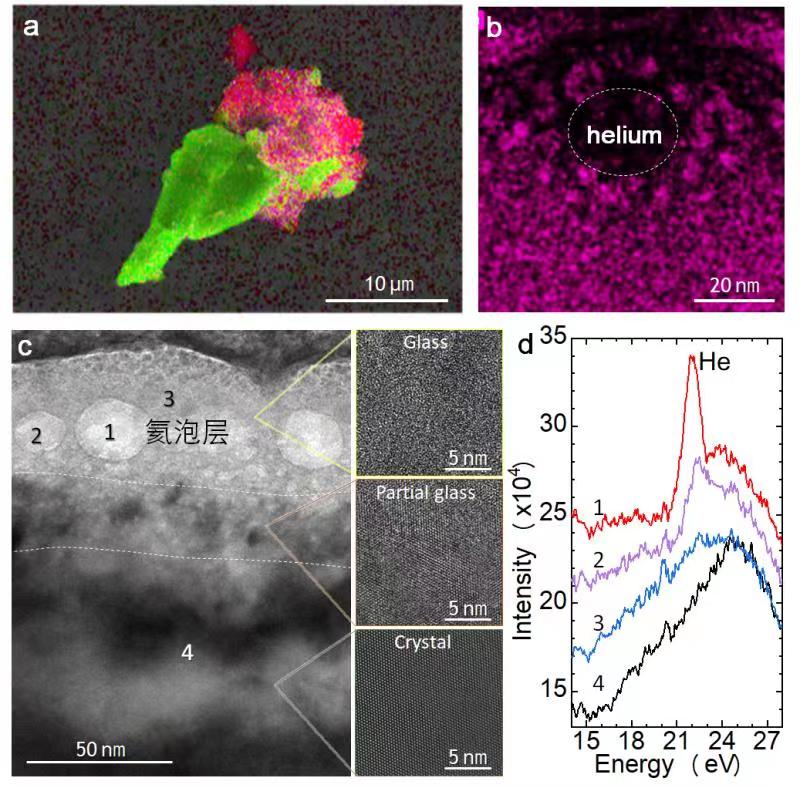
Fig.1 (a) EDS elemental mapping for a representative ilmenite (FeTiO3) particle with attachment of agglutinates. (b) A helium bubble observed under the transmission electron microscope. The red color shows the distribution of Fe element. (c) The bright field TEM image of the ilmenite (FeTiO3) sample. It has a glassy surface layer of about 50 nm in thickness where abundant helium bubbles are observed. (d) The electron energy loss spectrum curves at different positions in Fig. (c).
(Junqiang Wang)

