Metallic glass(also known as the amorphous alloy) is a new type of metal material that exhibits many excellent physical and chemical properties, such as good chemical catalytic activity (degradation of organic water pollutants, hydrogen production by electrolysis of water, etc.), excellent magnetic properties ( low coercive force, high magnetic permeability, large magnetic entropy change, etc.), excellent mechanical properties (high strength, high elasticity, high hardness, wear resistance, etc.) and strong corrosion resistance. According to the fundamental physical law, "water flows to the low," low energy is more stable, and the crystalline state is preferred for atom reconfiguration. Thus, obtaining the glassy disordered structure resulting from their higher energy is problematic. The rarity and ever-changing non-equilibrium metastable properties of glass mask its formation conditions, evolution laws, and physical nature. Among the 125 important scientific questions collected by Science, the great condensed matter physicist and Nobel Prize winner P.W. Anderson pointed out that "the nature of glass and glass transition may be the deepest and most interesting question in solid-state physics science; relevant research results are even of great significance to the development of intellectual problems such as neural networks, computer algorithms, evolution, and computational complexity."
Understanding the competitive relationship between crystal formation and glass formation during the preparation of metallic glasses is of great significance for improving the glass formation ability and controlling the quality and properties of glasses, which has been the focus of attention since the birth of metallic glasses for more than half a century. The essence of the metallic glasses has important guiding significance for regulating the non-equilibrium metastable characteristics of metallic glasses to improve their physical and chemical functional properties. In recent years, our team has studied the law and mechanism of glass formation and glassy state evolution in Au-based metallic glasses using high-precision flash differential scanning calorimetry (Flash DSC). A complete phase transition diagram of the metallic glass system during isothermal, cooling, and heating processes has been obtained for the first time. Based on this, the optimal method for preparing bulk ultrastable metallic glasses was proposed; two-step relaxation phenomena were found in the isothermal annealing process, and there were differences in the effects of beta relaxation and alpha relaxation on glass thermal stability and soft magnetic properties; Discovery of a correlation between glassy beta relaxation content and crystallization nucleation in supercooled liquids. This provides a theoretical basis for the preparation and performance regulation of metallic glasses. Related work was published in Acta Mater. 104, 25-32 (2016); Intermetallics 93, 101–105 (2018) and Acta Mater. 185, 38-44 (2020).
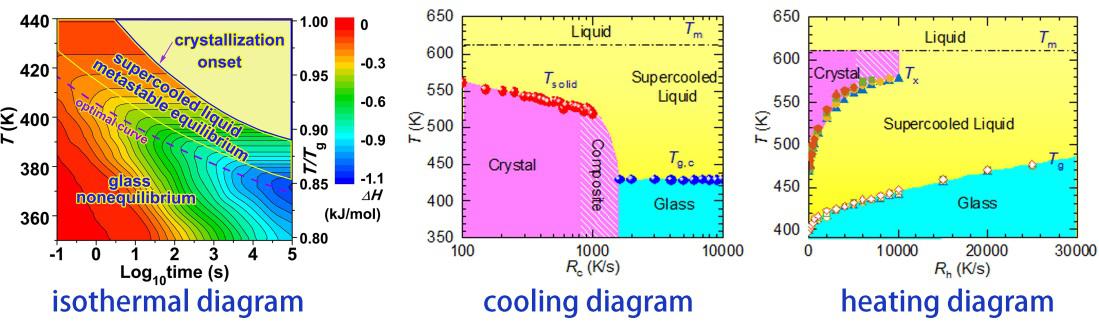
Fig. 1. Phase transition diagrams of metallic glass during isothermal, cooling and heating processes

