Metallic glasses, unlike crystalline alloys that are thermodynamically equilibrated, are metastable materials in far-from-equilibrium states. The advanced properties make metallic glasses promising candidates as functional materials, such as catalysts, biomaterials, soft magnetic materials, and magnetocaloric effects. In recent years, amorphous alloys such as Fe, Mg, and Co-based alloys have had excellent performance in discoloring azo dyes, with discoloration rates that can be tens or even hundreds of times higher than those of the corresponding crystalline alloys. However, the fading rate decreases rapidly as the pH of the solution increases. Considering that many of the actual dye effluents are alkaline solutions, it is important to develop amorphous alloy systems that can fade rapidly in alkaline solutions to facilitate their application.
In collaboration with Professor Wang Gang of Shanghai University, Pei-Pei Wang, a postgraduate student supervised by researcher Wang Junqiang of our team, found that Al-based amorphous alloys can rapidly degrade azo dyes over a wide pH range and that the degradation rate is 1.5 times and 189 times faster in alkaline (pH=12) and acidic (pH=2) solutions than in neutral conditions. The discoloration process of acidic and basic solutions largely conforms to the primary reaction mechanism. The fading in neutral solutions undergoes two processes: firstly, the degradation of the azo bond by the reduction of the amorphous alloy, and then the fading of the azo dye by the adsorption of the Al(OH)3 flocculent precipitate precipitated in the first stage. The adsorption of azo dyes by Al(OH)3 is a physical process with a much faster rate and more complete fading. A study of the surface structure and elemental composition of the alloy shows that the de-alloying reaction occurs in acidic and alkaline solutions, with the Al element reacting first and forming Ni and Y-rich nanoporous layers on the surface, and that this nanoporous structure can act to enhance dye adsorption and accelerate the dye fading degradation process. These results show that Al-based amorphous alloys have good prospects for application in dye effluent discoloration. The results were published in J. Alloys Compounds 701 (2017) 759。

Fig. 1. Azo dye solutions of different pH values all become completely transparent upon reaction with Al-based amorphous alloys.

Fig. 2. Decay of dye solution concentration with reaction time for different pH.
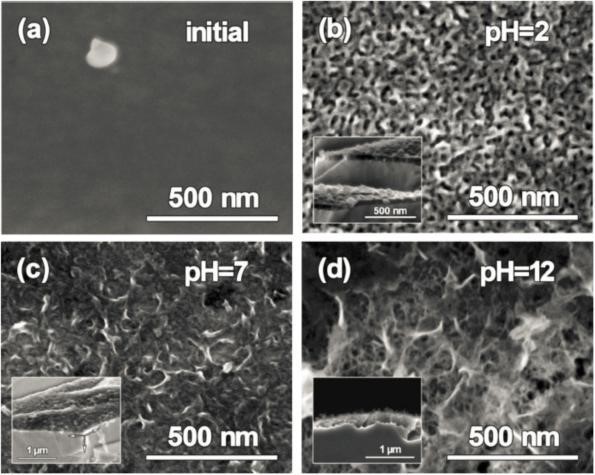
Fig. 3. Surface morphology of amorphous alloy strips before and after reaction: smooth surface before reaction, the nanoporous structure formed after the reaction.

