As a soft magnetic material, metallic glasses have broad application prospects in distribution transformers, motors, and inductors. However, preparing metallic glasses by rapidly cooling the melt is a non-equilibrium process that would result in high internal stress in the amorphous alloys, and processing strips into devices will also cause external stress. Due to the magnetoelastic interaction, stress makes the magnetic domain wall movement of amorphous alloy more complex, and the soft magnetic properties decrease, such as the decrease of magnetic permeability and the increase of coercive force. Generally, annealing near the glass transition temperature through atomic relaxation can release stress and reduce energy, thereby improving soft magnetic properties. Finding the optimal annealing conditions to achieve rapid and effective release of stress and lower energy states is a critical technical issue in promoting the application of amorphous alloys.
Pro. Ediger of the University of Wisconsin-Madison in the United States discovered ultra-low energy metallic glass by vapor deposition in 2007. It may take hundreds of years to obtain such a low-energy state through ordinary annealing and relaxation methods. This material is also called ultra-stable amorphous or ultra-stable glass. Our previous research found that the amorphous alloy thin film prepared by magnetron sputtering can also reach a low energy state and has a higher glass-liquid transition temperature, which is conducive to its use at higher temperatures; due to the common Changes in energy state and microstructure, the crystal growth rate of this ultra-stable amorphous alloy is slower during crystallization, which is conducive to the formation of more crystal nuclei. It provides a new idea for preparing nanocrystalline materials with more excellent properties [J.Q. Wang , et al. Acta Mater 79, 30 (2014)].
Vapor deposition can prepare amorphous alloys with ultra-low energy states, but the materials prepared by this method are all thin film samples, and its application is limited. Obtaining strips or bulk amorphous alloys designed by liquid cooling in a low-energy state has more practical significance for improving the performance of amorphous alloys and promoting their applications. Wang Junqiang, from the Magnetic Materials Division, in cooperation with Pro. Ediger and Academician Perepezko (from the University of Wisconsin-Madison, USA) systematically studied the Au-based amorphous material prepared by the liquid cooling method by simulating the influence of the substrate temperature and deposition rate during the vapor deposition process. The evolution law of the energy state of the alloy under different annealing temperatures and annealing times [J.Q. Wang, et al. Acta Mater 104,25 (2016)]. We found that: (1) there is an optimal annealing condition for the preparation of low-energy metallic glass: for a given annealing time, there is an optimal annealing temperature. For the gold-based amorphous alloy model system studied in this work, the relationship between the optimal annealing temperature (Ta) and the annealing time (ta) is: Ta = 274+ 132/log(ta), see Figures 1-2. (2) the amorphous alloy has experienced three states During the annealing process: non-equilibrium state (glass state), metastable equilibrium state (supercooled liquid state), and crystallization process, as shown in Figure 2. (3) the nonlinear relationship between the thermodynamic stability (inversely proportional to the energy state) and the kinetic stability (proportional to the glass-liquid transition temperature) of metallic glass: beta relaxation increases the thermodynamic stability. At the same time, the kinetic stability decreases; while the alpha relaxation increases the thermodynamic equilibrium, the kinetic strength also increases, as shown in Figure 3.
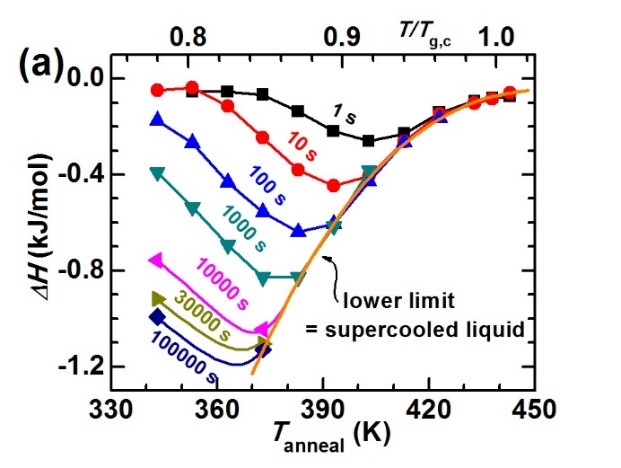


Figure 1. (a) The relationship between the decreased energy state (enthalpy) of the amorphous alloy and the annealing temperature; (b) The energy state of the amorphous alloy decreases with the annealing time; (c) The energy state of the fully relaxed amorphous alloy reaches extension of the subcooled liquid
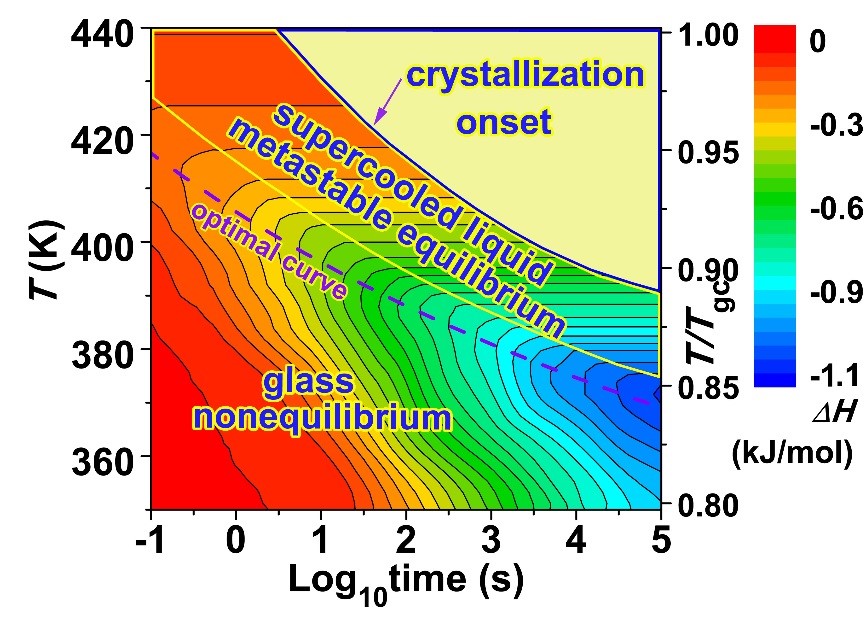
Figure 2. Two-dimensional contour plot of energy state (enthalpy) as a function of annealing temperature and annealing time. Adjacent contour lines from red to blue represent an energy reduction of 50 J/mol; from lower left to upper right can be divided into three regions: non-equilibrium state (glass), metastable equilibrium state (supercooled liquid), and crystallization process; The dotted line represents the optimal annealing conditions for the preparation of low-energy state amorphous alloys.
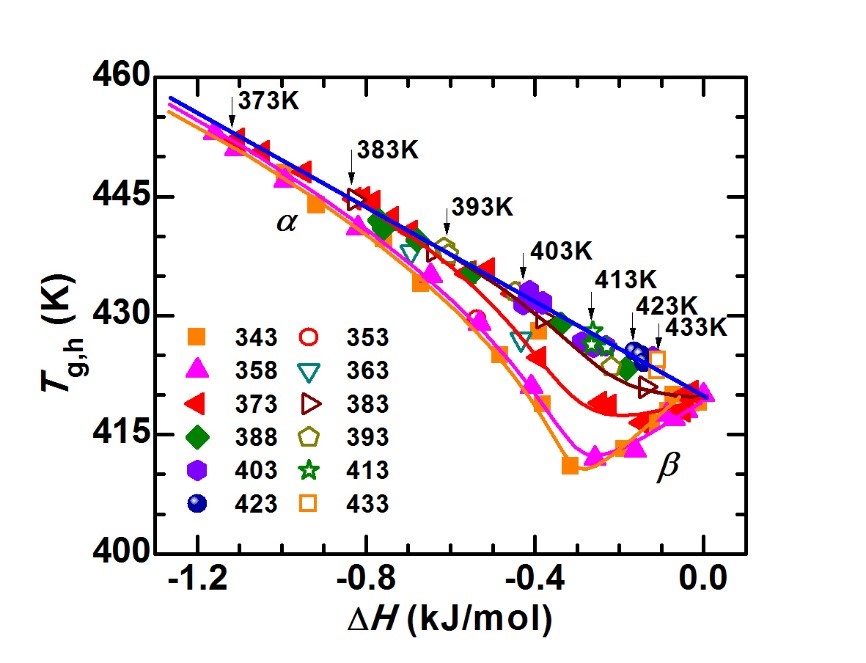
Figure 3. The relationship between the glass-liquid transition temperature (Tg,h) and the decrease of enthalpy (△H) during annealing at different temperatures

